MBI Explained
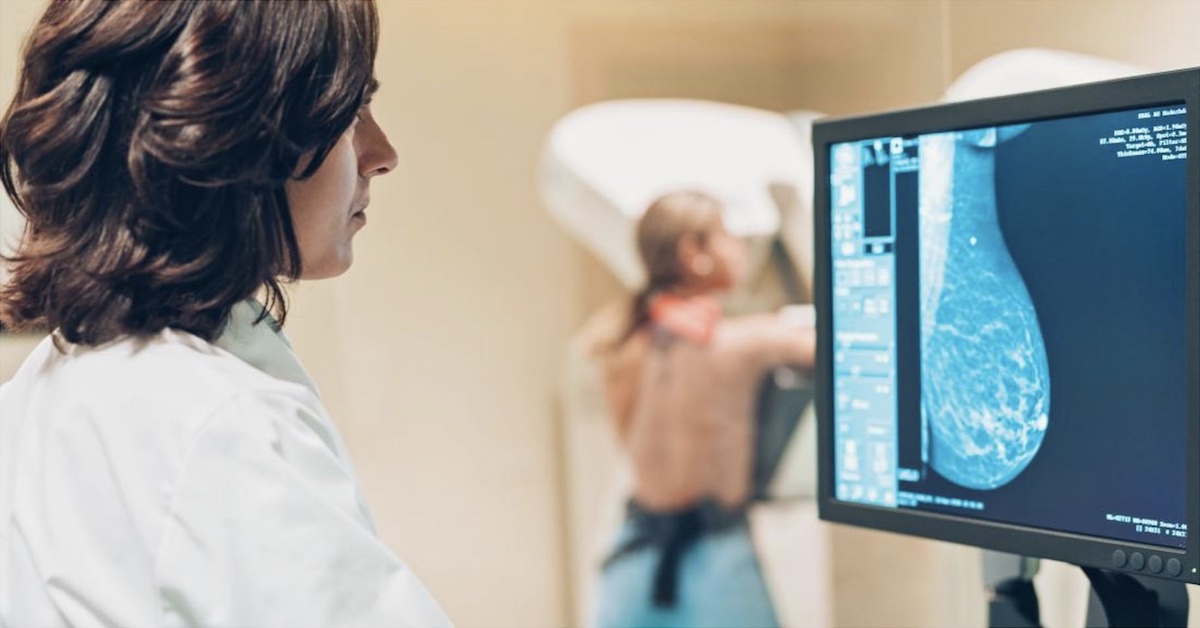
Molecular breast imaging (MBI) has been referred to as both MBI and breast-specific gamma imaging (BSGI), depending on the type of camera used. For consistency, the term MBI will be used to refer to these techniques, which use small field-of-view gamma cameras designed specifically for breast imaging using technetium-99m sestamibi.
| License Data | US FDA TECHNETIUM TC-99M SESTAMIBI |
| Route of Administration | Intravenous |
| ATC Code | VO9GA01 (WHO) |
| Formula | C36H66N6O6Tc |
In 1997, the Food and Drug Administration (FDA) approved the use of a radiopharmaceutical, technetium-99m sestamibi (Miraluma) for scintimammography, which used a conventional gamma camera to image the breast. Technetium-99m sestamibi, showed a high propensity to accumulate in breast tumors, which allowed for “functional imaging” not affected by anatomic characteristics such as breast density or surgical distortion. There are two mechanisms by which Technetium-99m sestamibi accumulates in breast tumors. The first is that it binds preferentially to the mitochondria in cells; since cancers have a high density of mitochondria (which is a marker for proliferative activity) they accumulate more technetium-99m sestamibi than the adjacent normal tissue.1 Secondly, breast tumors produce significant neoangiogenesis that enables increased uptake of the radiopharmaceutical.2
The use of cadmium zinc telluride (CZT) direct conversion detectors showcase a technology upgrade in nuclear medicine where photon energy released by a radiopharmaceutical, such as technetium-99m sestamibi, is directly converted into electric signals allowing for accurate identification of event location and energy. With computer and software technology, complex calculations can be performed very quickly to convert the detected radiation into images and information that is useful for radiologists.
LOW BREAST RADIATION RISK COMPARED TO MAMMOGRAPHY
Optimizations in detector design, patient preparation, and radiopharmaceutical delivery have reduced the administered activity from 740–1,100MBq (20–30 mCi) to the current off-label standard of 240–300MBq (6.5–8 mCi).5, 6 The average absorbed radiation dose to the breast from 300MBq (8 mCi) of 99mTc-sestamibi is estimated to be 1.1mGy, compared with 3.0–4.5mGy that is associated with mammography and breast tomosynthesis (3D mammography).7
Since 99mTc-sestamibi is systemically distributed, tissues outside the breast receive the largest radiation dose. The estimated effective (whole-body) dose for 300MBq (8 mCi) of 99mTc-sestamibi is 2.1–2.6 mSv, which is at, or lower than, annual natural background levels (~3 mSv).8 For reference, the effective dose of chest CT can approach 6 mSv.9 Tissues with the highest exposures include the colon (7.1mGy), urinary bladder (3.2mGy), and gallbladder (11.5mGy).10 Various organizations assessing radiation risk and radiation protection (Health Physics Society, American Association of Physicists in Medicine, International Organization for Medical Physics, and United Nations Scientific Committee on the Effects of Atomic Radiation) state that risks from radiation doses of less than 100 mSv are not significantly different from zero.8 Thus, current MBI radiation exposure is deemed to pose negligible risk to the patient, with minimal theoretic risk of inducing cancer in any of these organs.8 The administered activity of 99mTc-sestamibi for MBI continues to decrease with technologic advancement. Tao et al. showed that new image-processing algorithms maintain lesion conspicuity with a simulated half-dose (150MBq [4 mCi]) injection.11 Furthermore, continued advances in CZT module design will improve sensitivity and should allow further dose reduction.
CLINICAL PEARLS

MBI has high sensitivity (95%) and specificity (80%) for detecting malignancy without a simultaneous increase in false-positive rates, suggesting its suitability for screening women with dense breasts.12

MBI in women with dense breasts shows better sensitivity compared with mammography, and improved specificity compared with both ultrasound and magnetic resonance imaging (MRI).13, 14

MBI can be used as a problem-solving tool for the evaluation of clinical indications following complex mammography or ultrasound, or for unexplained physical findings.15
MBI PRODUCTS
SmartBreast Corporation’s initial products are scanners, using molecular breast imaging (MBI) technology. Our scanners are manufactured under the brand name Eve Clear Scan™, which along with SmartBreast™ and the EVE owl logo, are trademarks of SmartBreast Corporation.
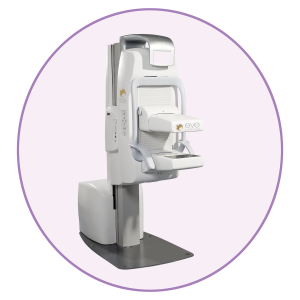
Eve Clear Scan™ model e750 uses solid state cadmium-zinc-telluride (CZT) technology and compact dual detector design to allow for up- close breast imaging to the chest wall. The result is improved lesion detection, intrinsic resolution, spatial resolution, sensitivity, and degradation-free image uniformity.
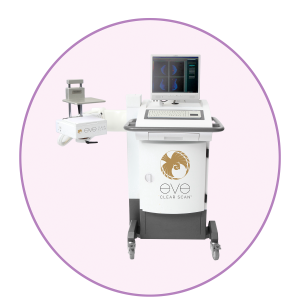
Eve Clear Scan™ model e680 uses a compact sodium iodide (NaI) single detector design, optimized for breast specific gamma imaging (BSGI). It is a portable system, which enables the user to move and position the machine in a desired location.
Apart from our MBI scanners, we will support breast health providers with the Eve Suite™ of dense-breast solutions, which include a cohesive combination of AI software (artificial intelligence), and CDS software (clinical decision support).
CLINICAL IMAGES
High Risk Screening
MBI can be used as a supplemental imaging tool for women with dense breasts and who are at intermediate or high risk for breast cancer based on risk assessments tools, such as the Gail model or the Tyrer-Cuzick model.
A 47-year-old asymptomatic female with heterogeneously dense breasts and a family history of breast cancer – her mother was diagnosed with Stage 4 breast cancer. Gail Score showed a 21% lifetime risk of developing breast cancer and underwent MBI for high-risk screening. Mediolateral oblique (A) and cranio-caudal (B) molecular breast images (open arrow) demonstrate a focal uptake at the upper outer quadrant of the left breast. This was co-localized on ultrasound (C) as an irregular hypoechoic mass (open arrow) with guided biopsy revealing invasive ductal carcinoma.
Assessing for Disease Extent and Bilaterality of Lesions
MBI can be utilized to guide preoperative surgical planning either to assess additional findings on mammography and/or ultrasound or to pick up potential lesions that are occult in anatomic-based imaging.
A 44-year-old female with moderate mass uptake at the upper outer right breast as seen on the cranio-caudal (A) and mediolateral oblique (B) molecular breast images. This corresponded to a large solid mass that was considered to be a giant fibroadenoma vs. phyllodes tumor. In the left breast, moderate pathologic uptake of contiguous masses in segmental distribution at the mid lower aspect as seen on mediolateral oblique (C) and cranio-caudal (D) molecular breast images. She had histopathologically proven invasive ductal carcinoma.
Evaluation of Disease Extent
MBI can be useful for assessing tumor extent and how this information can implicate treatment and surgical options.
A 47-year-old female with diffuse moderate pathologic uptake involving almost the entire left breast as seen on the mediolateral oblique (A), and cranio-caudal (B) molecular breast images and histopathologically proven Her2 (+) invasive mammary carcinoma. She initially underwent neoadjuvant chemotherapy (NAC) followed by mastectomy with staged breast reconstruction using a tissue expander. Final histopathology showed multifocal disease.
A 59-year-old female with moderate multifocal uptake in the left breast. Mediolateral oblique (A) and cranio-caudal (B) molecular breast images showed two masses in same quadrant – at the lower outer/5:00 axis and at the mid lower/6:00 axis. Post-lumpectomy specimen mammogram (C) revealed that the 5:00 axis lesion was ductal carcinoma in situ, while the 6:00 axis lesion turned out to be invasive ductal carcinoma.
MBI LEXICON
A lexicon for the description of MBI images based on the familiar Breast Imaging Reporting and Data System (BI-RADS) terminology for other diagnostic technology such as mammography, ultrasonography and MRI, was developed in 2012.16 This allows for effective standardized reporting and communication of breast imaging findings and recommendations. For more details, click here.
These are the definitions of lesion intensity provided to observers for use during the interpretation task. However, it is recommend that lesion intensity be judged relative to subcutaneous fat (rather than relative to background uptake) for greater consistency.
References
1 L Delmon-Moingeon, R Piwnica-Wo, AD Van den Abbeele, BL Holman, A Davison, AG Jones. Uptake of the cation hexakis (2-methoxyisobutylisonitrile)-technetium-99 m by human carcinoma cell lines in vitro. Cancer Res. 1990; 50(7): 2198–202.
2 S Sharma, MC Sharma, C Sarkar. Morphology of angiogenesis in human cancer: a conceptual overview, histoprognostic perspective and significance of neoangiogenesis. Histopathology. 2005; 46 (5): 481–9.
3 O'Connor M, Rhodes D, Hruska C. Molecular breast imaging. Expert Review of Anticancer Therapy. 2009; 9(8): 1073-1080.
4 Taillefer R. The role of 99mTc-sestamibi and other conventional radiopharmaceuticals in breast cancer diagnosis. Seminars in Nuclear Medicine. 1999; 29(1): 16-40.
5 Shermis RB, Wilson KD, Doyle MT, et al. Supplemental breast cancer screening with molecular breast imaging for women with dense breast tissue. AJR. 2016; 207: 450–457.
6 Dibble EH, Hunt KN, Ehman EC, O’Connor MK. Molecular breast imaging in clinical practice. AJR. 2020; 215: 277–284.
7 Hruska CB. O’Connor MK. Curies, and Grays, and Sieverts, Oh My: A Gide for Discussing Radiation Dose and Risk of Molecular Breast Imaging. J Am Coll Radiol. 2015 Oct; 12(10): 1103–1105.
8 Hruska CB. Let’s get real about molecular breast imaging and radiation risk. Radiol Imaging Cancer. 2019; 1: e190070.
9 Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008; 248: 254–263.
10 Mattsson S, Johansson L, Leide Svegborn S, et al. Radiation dose to patients from radiopharmaceuticals: a compendium of current information related to frequently used substances. Ann ICRP. 2015; 44: 7–321.
11 Tao AT, Hruska CB, Conners AL, et al. Dose reduction in molecular breast imaging with a new image-processing algorithm. AJR. 2020; 214: 185–193.
12 Sun Y, Wei W, Yang HW, Liu JL. Clinical usefulness of breast-specific gamma imaging as an adjunct modality to mammography for diagnosis of breast cancer: a systemic review and meta-analysis. European Journal of Nuclear Medicine and Molecular Imaging. 2013; 40(3): 450-463.
13 Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography versus mammography alone in women at elevated risk of breast cancer. JAMA. 2008; 299(18): 2151-2163.
14 O'Connor M. Molecular breast imaging: an emerging modality for breast cancer screening. Breast Cancer Management. Volume 4, No. 1. 7, 2015
15 Shermis RB, Refern RE, Burns J, Kudrolli H. Molecular Breast Imaging in Breast Cancer Screening and Problem Solving. Radiographics. Sep-Oct 2017; 37(5): 1309-1606.
16 AL Conners, CB Hruska, CL Tortorelli, RW Maxwell, DJ Rhodes, JC Boughey, WA Berg. “Lexicon for standardized interpretation of gamma camera molecular breast imaging: observer agreement and diagnostic accuracy.” Eur J Nucl Med Mol Imaging (2012) 39:971–982. DOI 10.1007/s00259-011-2054-z
