Our 3C’s To Combat The Big C

Certainty
Annual mammography screening between ages 40 and 75 is the highest standard of care in Western industrialized countries, but there is a deadly secret that has only come into public awareness in the past decade or so -- mammography often fails in dense breasts!
The Problem of Uncertainty
Breasts are composed of fatty, connective, and fibro-glandular tissue types. Dense breasts have a higher proportion of fibro-glandular and connective tissue relative to fatty breasts. According to the Breast Imaging-Reporting and Data System (BI-RADS) classification, the levels of density are: A: almost entirely fatty (about 10% of women), B: scattered areas of fibro-glandular density (about 40% of women), C: heterogeneously dense (about 40% of women), and D: extremely dense (about 10% of women). Women with significant amounts of fibro-glandular tissue, typically those rated as C and D on mammography, are classified as having “dense breasts.”
Breast Imaging-Reporting and Data System (BI-RADS)
Classification of Breast Density
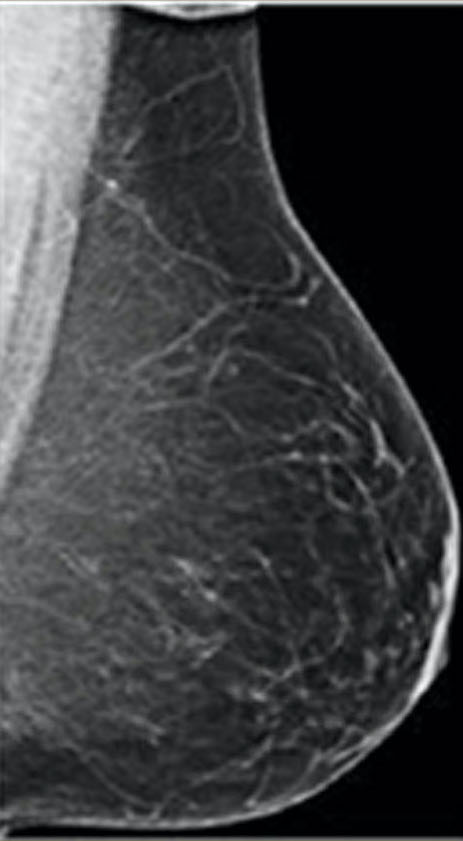
almost entirely fatty
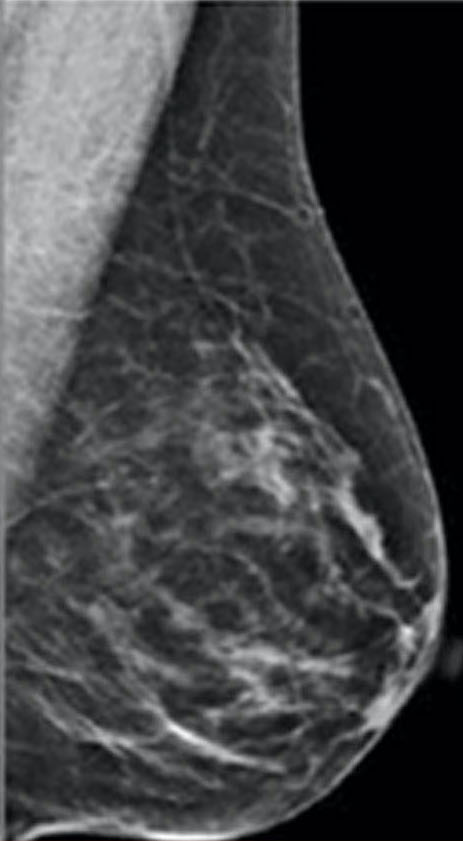
scattered areas of fibro-glandular density
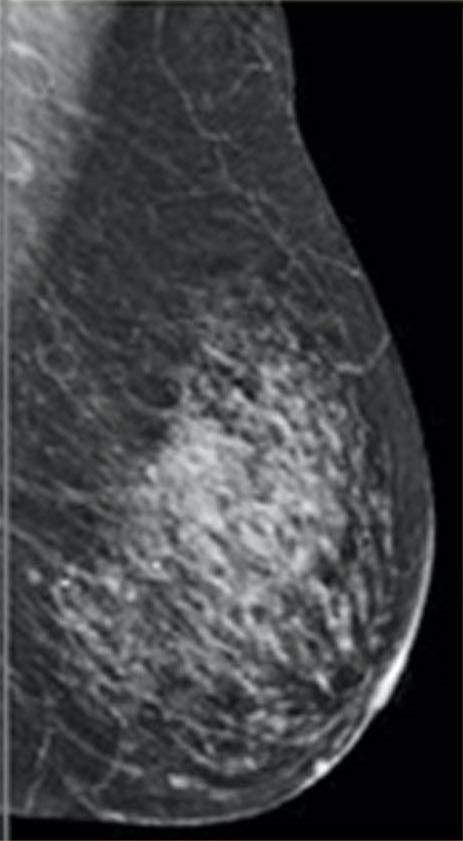
heterogeneously dense
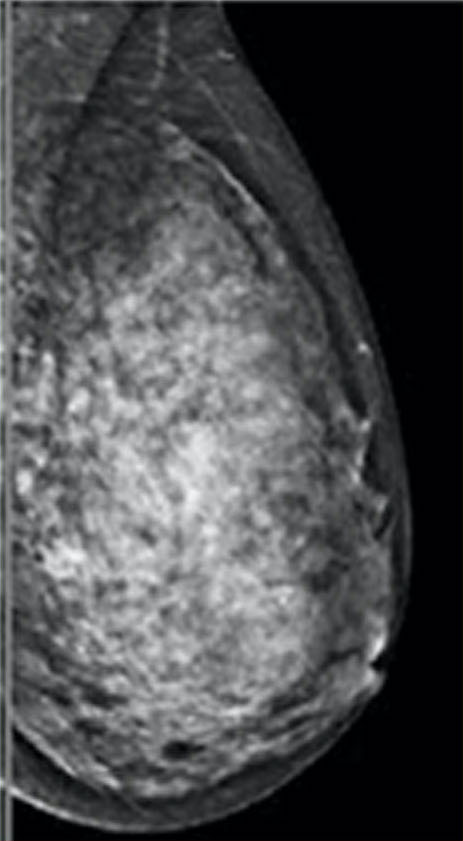
extremely dense

Increased breast density renders mammography a far less sensitive tool for early cancer detection.1
40% of Western women2 and 70% of Asian women have dense breasts.3

Mammography and digital breast tomosynthesis (DBT or “3D-mammography”), fail to reveal cancer about half of the time when tumors are small and easily treatable.2

Mammograms of dense breasts with cancer cells often look “normal”, failing to identify cancer until such time that a large tumor becomes obvious, and more likely to have spread throughout the body.
Seeing the Unseen
With screening mammography, “dense breasts” can hide cancer tumors in the midst of similarly dense fibro-glandular tissue. Because of this, a supplemental imaging tool is necessary. The medical imaging community has offered several partial solutions:
Ultrasound
Digital Breast
Tomosynthesis
(DBT / 3D-mammography)
Contrast-enhanced
Spectral
Mammography
Magnetic
Resonance
Imaging
Molecular
Breast
Imaging
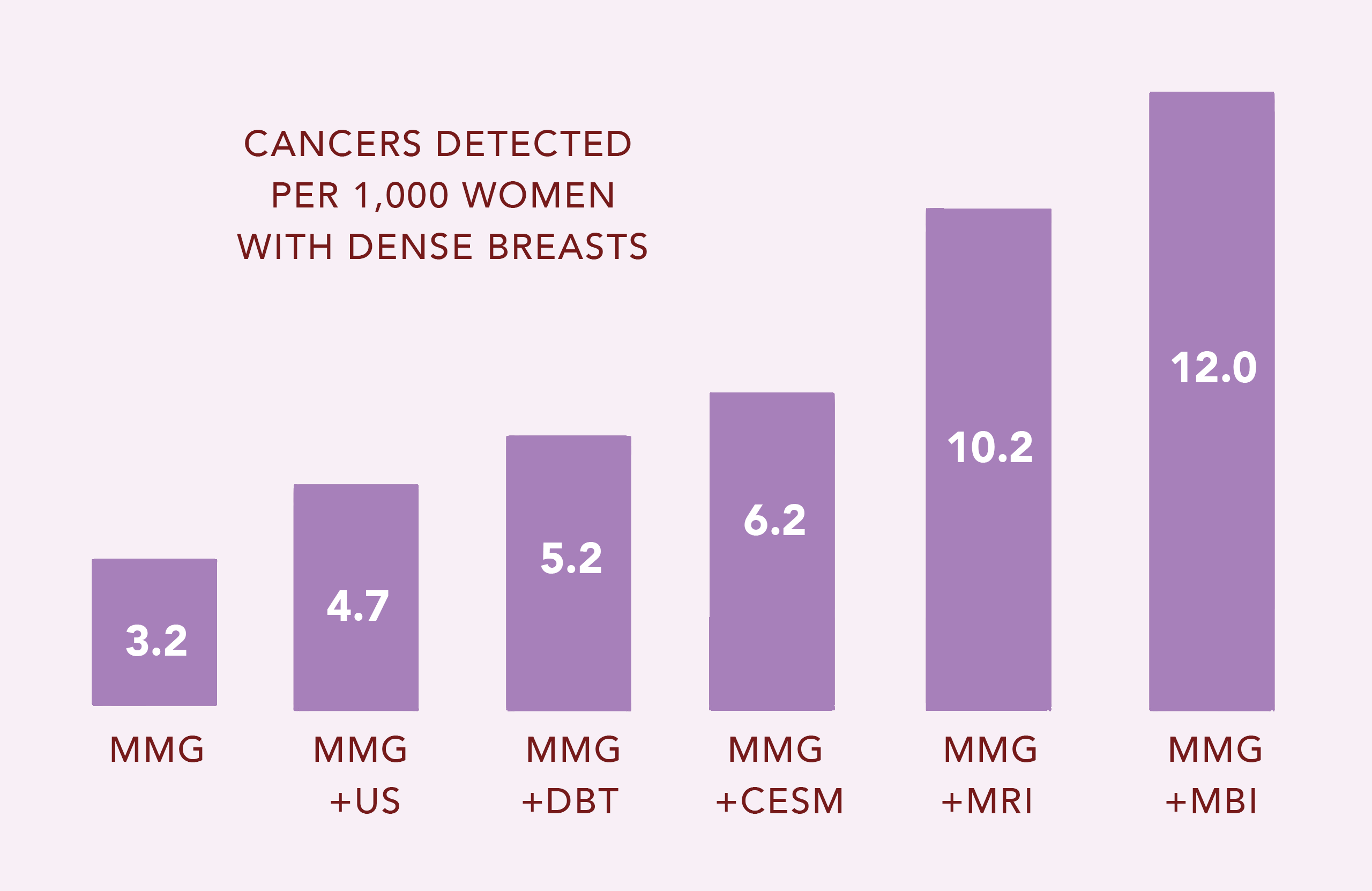
The chart shows that mammography (MMG) finds only 3.2 cancers per 1,000 women with dense breasts. MBI identifies 8.8 additional cancers that were missed by MMG! Ultrasound (US), digital breast tomosynthesis (DBT) and Contrast-enhanced spectral mammography (CESM) detect only additional 1.5, 2.0 and 3.0 of the missed cancers, respectively. MRI and MBI are the most effective ways to find those cancers missed by MMG, DBT, CESM and US.5-9
While both MRI and MBI have sensitivities > 90% (they find almost all cancer), MBI alone has specificity > 90% (almost everything MBI finds is truly cancer). The specificity of MRI is much lower, at about 50% (many false positive findings; only half of what MRI finds is truly cancer). Thus, MRI results in numerous unnecessary biopsies that prove to be negative. MRI is also 3X more expensive than MBI.10-13
References
1 Checka, Chun, Schnabel, et.al. “The Relationship of Mammographic Density and Age: Implications to Breast Cancer Screening.” American Journal of Roentgenology. 2012; 198: W292-W295.
2 Sprague, Gangnon, Burt, et al. “Prevalence of Mammographically Dense Breasts in the United States.” J Natl Cancer Inst (2014) 106:1-6.
3 Jong-Myon Bae, et.al. “Breast Density and Risk of Breast Cancer in Asian Women: A Meta-analysis of Observational Studies.” J Prev Med Public Health. 2016 Nov; 49(6): 367–375.
4 Mandelson, Oestreicher, et.al. “Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers.” J Natl Cancer Inst 2000 Jul 5;92(13):1081-7.
5 WA Berg, AI Bandos, EB Mendelson, et al., ”Ultrasound as the Primary Screening Test for Breast Cancer: Analysis from ACRIN 6666,” J Natl Cancer Inst (2016) 108:1-8
6 WA Berg, Z Zhang, D Lehrer, et al. “Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk,” JAMA (2012) 307:1394-1404.
7 S Hofvind, T Hovda, AS Holen, et al., “Digital Breast Tomosynthesis and Synthetic 2D Mammography versus Digital Mammography: Evaluation in a Population-based Screening Program,” Radiology (2018) 287:787-794.
8 S Ciatto, N Houssami, D Bernardi, et al., “Integration of 3D Digital Mammography with Tomosynthesis for Population Breast-Cancer Screening (STORM): A Prospective Comparison Study,” The Lancet/Oncology 14:583-589.
9 CB Hruska, AL conners, KN Jones, et al., “Diagnostic Workup and Costs of a Single Supplemental Molecular Breast Imaging Screen of Mammographically Dense Breasts,” Am J Roentgenol (2015) 204:1345-1353.
10 WA Berg, AI Bandos, EB Mendelson, et al. ”Ultrasound as the Primary Screening Test for Breast Cancer: Analysis from ACRIN 6666,” J Natl Cancer Inst (2016) 108:1-8
11 WA Berg, Z Zhang, D Lehrer, et al. “Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk.” JAMA (2012) 307:1394-1404.
12 S Hofvind, T Hovda, AS Holen, et al., “Digital Breast Tomosynthesis and Synthetic 2D Mammography versus Digital Mammography: Evaluation in a Population-based Screening Program.” Radiology (2018) 287:787-794.
13 S Ciatto, N Houssami, D Bernardi, et al., “Integration of 3D Digital Mammography with Tomosynthesis for Population Breast-Cancer Screening (STORM): A Prospective Comparison Study,” The Lancet/Oncology 14:583-589.
